Common the number and dimension of particles in Every single locale. Then take your area averages and locate their typical, so you've the general ordinary dimensions and quantity of particles for your cleanroom.
Layout a structure which allows for simple cleansing and routine maintenance, with obvious separation amongst distinctive method areas.
Cleanrooms can range between the incredibly modest to your pretty significant. Within the just one hand, just one-consumer laboratory can be crafted to cleanroom criteria in a number of sq. meters, and on the other, overall production facilities could be contained in a cleanroom with manufacturing facility flooring masking A huge number of sq. meters.
Just one specific version of the hardwall cleanroom can be a prefabricated cleanroom that has hardwalls, is cellular, and can be create quickly and easily.
Thoroughly clean rooms apply gowning protocols to forestall contamination from folks & clothing and to maintain the Class one hundred cleanliness classification.
He has loaded understanding and provides valuable insights and facts by his content and material on Pharmaguddu.com. For more inquiries or collaborations, please don’t wait to reach out by means of email at Call@pharmaguddu.com.
Suitable airflow is essential for carrying absent particles and contaminants and maintaining a controlled environment conducive to sterile product or service manufacturing.
These extremely-clean up environments are generally used in very sensitive industries like semiconductor production and nanotechnology exploration. Every component, from what is class 100 air filtration to gowning treatments, is meticulously managed to maintain this pristine state.
To guarantee worldwide harmonization and consistency, cleanroom classifications in cGMP recommendations normally align with Global expectations including ISO Regular. Let’s Examine cleanroom classifications according to Annex 1 and ISO conventional:
Areas encompassing the core manufacturing area are often known as supporting areas. These supporting areas have distinct functions as storage of in-process supplies, cleaned machines, content transfer and so forth.
As outlined by FDA, the stress differential need to be no less than 0.05 inch of drinking water. At the opening of doorway, the air ought to click here stream from the higher cleanliness room to decrease to avoid the doorway in the contamination.
This usually involves deciding upon the correct elements, structure, and HVAC system. Professional consultation is key in any cleanroom venture. Achieve out to Allied Cleanrooms to acquire a no cost quotation and session on your task.
Formulators of parenteral medicines need to be careful of unique concerns and issues that occur during growth and manufacture.
Right here at Lighthouse All over the world Solutions, we’re all about just that: answers. Answers on your particle counting demands, remedies for serving to you obtain your cleanroom classification, and remedies to the ups and downs that come with working inside of a cleanroom.
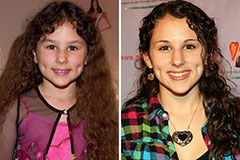 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Hailie Jade Scott Mathers Then & Now!
Hailie Jade Scott Mathers Then & Now! Freddie Prinze Jr. Then & Now!
Freddie Prinze Jr. Then & Now! Elisabeth Shue Then & Now!
Elisabeth Shue Then & Now! Monica Lewinsky Then & Now!
Monica Lewinsky Then & Now!